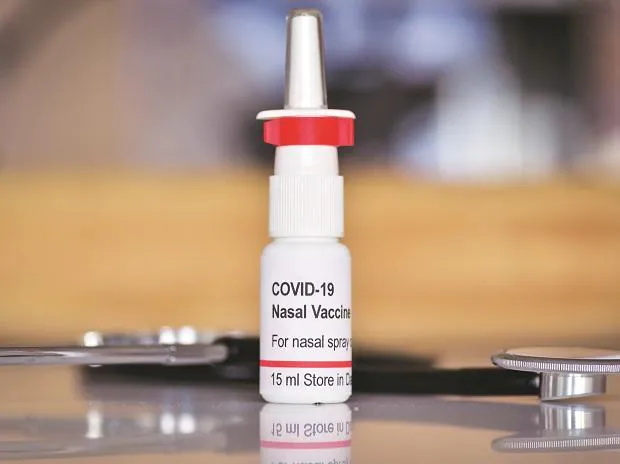
On 26th January 27, 2023, Bharat Biotech launched the world’s first intranasal vaccine against Covid 19 in New Delhi. The vaccine uses a modified chimpanzee adenovirus to carry the Covid spike protein and cannot replicate in the body. It was developed with the support of the Department of Biotechnology and through a partnership between Bharat Biotech and Washington University- St Louis.
By Amb Satish Chandra
The Department of Biotechnology and its public sector unit the Biotechnology Industry Research Assistance (BIRAC) funded the product development and clinical trials. The Washington University developed the vector carrying the spike protein and evaluated it in preclinical trials, and Bharat Biotech handled the product development and manufacturing.
Some experts contend that an intranasal vaccine may provide better protection against infection as it generates immunity in the mucosal membrane notably in the nose and mouth. It certainly scores over the injectable vaccine both in terms of ease of administration and cost as it does not require needles, syringes, alcohol wipes, band aids, skilled personnel etc to administer it, and does not generate biomedical waste.
The vaccine has undergone extensive Phases I, II and III clinical trials in several sites in India with successful results. The nasal delivery system has been designed and developed to be cost-effective in low- and middle-income countries. This vaccine is stable at 2-8°C for easy storage and distribution. Large manufacturing capabilities have been established by Bharat Biotech at multiple sites across India, including Gujarat, Karnataka, Maharashtra, and Telangana, with operations pan India.
Unlike the one shot injectable vaccine the nasal vaccine is to be administered in two doses with a four week interval comprising four drops in each nostril. It is, however, currently being rolled out as a onetime booster dose for those having been injected with Covaxin or Covishield.
The vaccine has the double benefit of enabling faster development of variant-specific vaccines and easy nasal delivery that enables mass immunization to protect from emerging variants of concern. It promises to become an important tool in mass vaccinations during pandemics and endemics.
It is in the context of the foregoing that Mr. Mansukh Mandviya on the occasion of the launch of this vaccine asserted that it constituted a “historic achievement and a testimony to the innovative zeal of our scientists.”
This article first appeared in www.vifindia.org and it belongs to them.